Research Bald Lab
Decoding Tumor Immunology
Neutrophils: Friend of Foe?
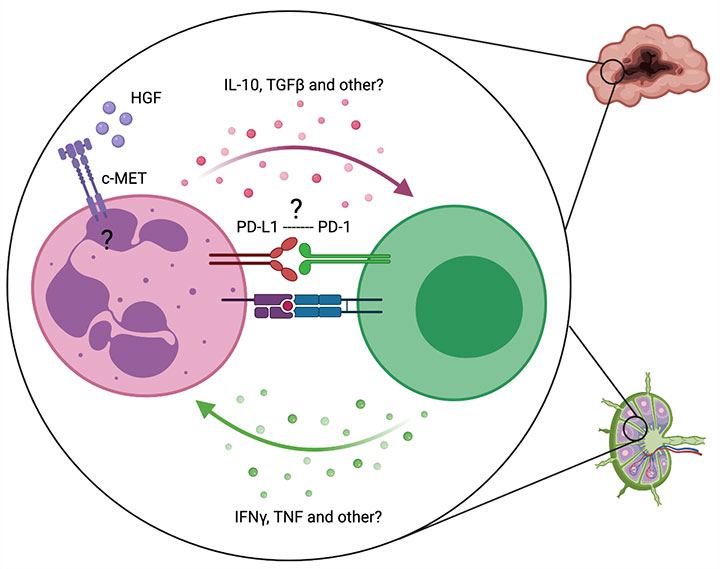
Neutrophils are the most abundant immune cells in the human body. However, our knowledge about their role in cancer progression and therapy-resistance is limited.We have recently shown, that upon treatment with T cell-based immunotherapies neutrophils are recruited into the TME and tumor-draining lymph nodes. Here, they acquire an immune-suppressive phenotype, impairing T cell function and limiting the efficacy of immunotherapies (Glodde et al., 2017).
In this project, we aim to understand the molecular mechanisms leading to the recruitment of neutrophils, driving their phenotypic plasticity and are involved in regulating T cell function, in order to develop novel neutrophil-targeting cancer immunotherapies.
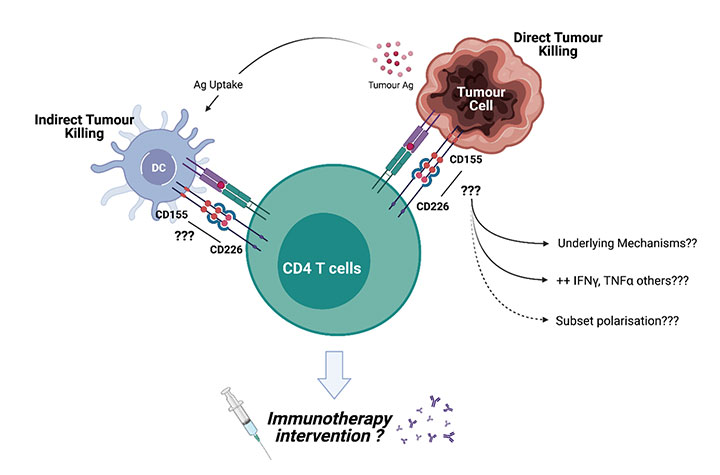
CD226+ CD4 T cells’ Hidden Talents?
T cell function is regulated by signals through T cell activating and inhibitory receptors. We have recently, shown that CD226 is important for the fitness of tumour-infiltrating CD8+ T cells and the efficacy of cancer immunotherapies (Braun et al., 2020).In this project, we aim to understand the relevance of CD226 for CD4+ T cell differentiation and function in infectious diseases and cancer.
CD226+ CD4 T cells’ Hidden Talents?
T cell function is regulated by signals through T cell activating and inhibitory receptors. We have recently, shown that CD226 is important for the fitness of tumour-infiltrating CD8+ T cells and the efficacy of cancer immunotherapies (Braun et al., 2020).In this project, we aim to understand the relevance of CD226 for CD4+ T cell differentiation and function in infectious diseases and cancer.
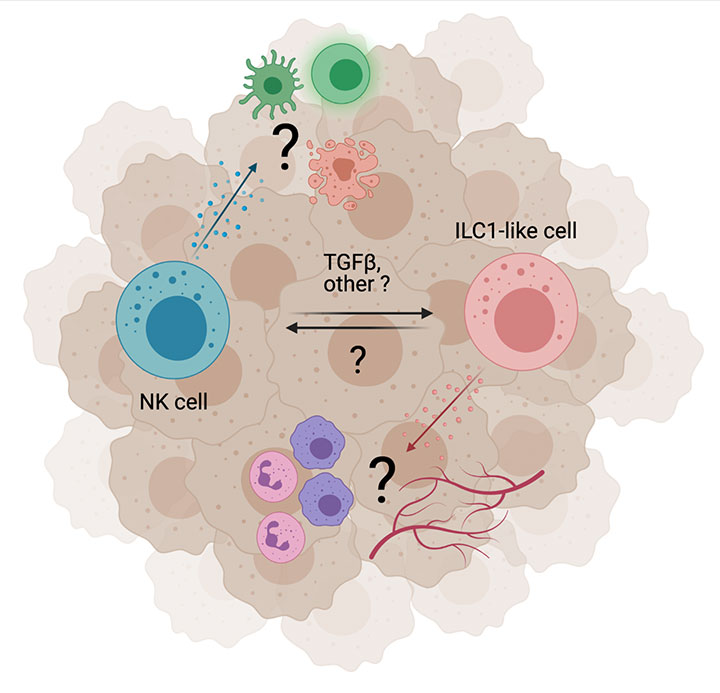
Innate Lymphoid Cell Plasticity
To quickly adapt to changes in their environment, immune cells are highly plastic. However, the underlying mechanisms and the importance of NK cell plasticity for the development, progression and efficacy of immunotherapies in cancer remains elusive.We have recently shown that TGFβ-signaling drives phenotypic plasticity of NK cells in the TME (Gao et al. 2017). We observed that NK cells acquired an ILC1-like phenotype, which led to the immune escape of mouse tumors. Based on these findings, we seek to (A) further understand the cellular and molecular mechanisms contributing to NK cell plasticity and (B) advance our knowledge on the importance of ILC1-like cells for angiogenesis and myeloid cell recruitment in cancer.
Trying to find more Hidden Talents? Join the ride!
Interested in more information about our projects?
Further Reading
CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8+ T Cells.
Braun M, Aguilera AR, Sundarrajan A, Corvino D, Stannard K, Krumeich S, Das I, Lima LG, Meza Guzman LG, Li K, Li R, Salim N, Jorge MV, Ham S, Kelly G, Vari F, Lepletier A, Raghavendra A, Pearson S, Madore J, Jacquelin S, Effern M, Quine B, Koufariotis LT, Casey M, Nakamura K, Seo EY, Hölzel M, Geyer M, Kristiansen G, Taheri T, Ahern E, Hughes BGM, Wilmott JS, Long GV, Scolyer RA, Batstone MD, Landsberg J, Dietrich D, Pop OT, Flatz L, Dougall WC, Veillette A, Nicholson SE, Möller A, Johnston RJ, Martinet L, Smyth MJ, Bald T.
Immunity. 2020 Oct 13;53(4):805-823.e15.
Immunity. 2020 Oct 13;53(4):805-823.e15.
The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies.
Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O'Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, Hinze D, Rogava M, van der Sluis TC, Ruotsalainen JJ, Gaffal E, Landsberg J, Ludwig KU, Wilhelm C, Riek-Burchardt M, Müller AJ, Gebhardt C, Scolyer RA, Long GV, Janzen V, Teng MWL, Kastenmüller W, Mazzone M, Smyth MJ, Tüting T, Hölzel M
Immunity. 2017 Oct 17;47(4):789-802.e9.
Immunity. 2017 Oct 17;47(4):789-802.e9.
Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy.
Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, Hinze D, Rogava M, van der Sluis TC, Ruotsalainen JJ, Gaffal E, Landsberg J, Ludwig KU, Wilhelm C, Riek-Burchardt M, Müller AJ, Gebhardt C, Scolyer RA, Long GV, Janzen V, Teng MWL, Kastenmüller W, Mazzone M, Smyth MJ, Tüting T, Hölzel M
Immunity. 2017;47(4):789-802.e9.
Immunity. 2017;47(4):789-802.e9.
Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells.
Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, Yan J, Bartholin L, Lee JS, Vivier E, Takeda K, Messaoudene M, Zitvogel L, Teng MWL, Belz GT, Engwerda CR, Huntington ND, Nakamura K, Hölzel M, Smyth MJ
Nat Immunol. 2017 Sep;18(9):1004-1015.
Nat Immunol. 2017 Sep;18(9):1004-1015.
Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma.
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, Tüting T
Nature. 2014 Mar 6;507(7490):109-13.
Nature. 2014 Mar 6;507(7490):109-13.