Research Glodde Lab
Decoding Tumor Immunology
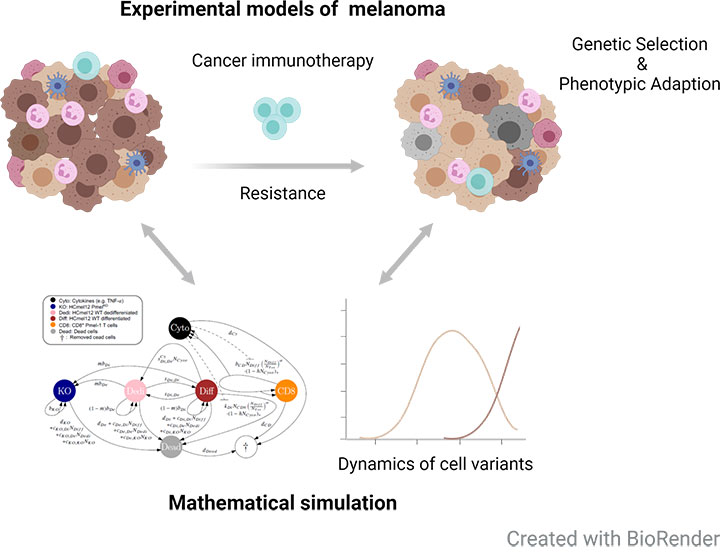
Combining experimental models of cancer immunotherapy with mathematical modelling
Immunotherapies have revolutionized the treatment of many cancer patients. However, the majority of patients does not respond or develop resistance after an initial response. One important resistance mechanism is the loss of antigen expression or impaired presentation. In collaboration with interdisciplinary research areas (Prof. Anton Bovier, IAM; Prof. Kevin Thurley, IEO; University of Bonn), we combine experimental and mathematical models of melanoma to better understand the evolutionary dynamics of genetic antigen loss variants and phenotypic adaptation in the context of adoptive T cell transfer immunotherapy (ACT) (Glodde et al., in rev.). These will be also extended by spatio-temporal simulations to decipher cellular interactions in distinct organs during cancer immunotherapy.Targeting the tumor microenvironment to improve cancer immunotherapy
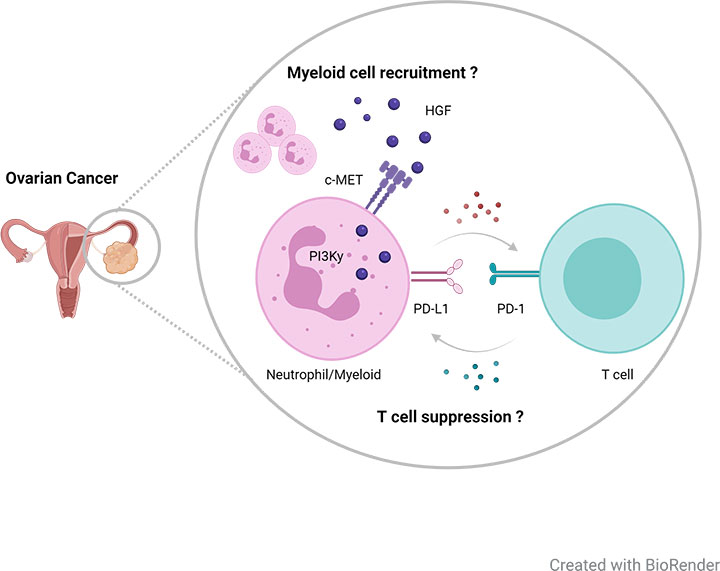
Resistance to immunotherapy can also be in part explained by the regulation and nature of the complex immune system. Recently, we found that immunotherapies induce a reactive recruitment of immunosuppressive neutrophils into tumor tissue, thereby limiting therapeutic efficacy. This recruitment was dependent on the receptor tyrosine kinase c-MET and blocking of c-MET signaling enhanced the efficacy of immunotherapies in a variety of cancer models (Glodde et al., 2017).Neutrophils also appear to play a critical role for disease progression and T cell suppression in advanced ovarian cancer. However, the underlying mechanism how these or other myeloid immune cells contribute to immune regulation and disease progression in ovarian cancer is poorly understood.
By using orthotopic ovarian cancer models carrying human-relevant mutations, we aim to provide a deeper understanding of myeloid pathobiology in order to identify and develop novel targeted and personalized treatment strategies for microenvironmental reprogramming in combination with immunotherapies.
Targeting the tumor microenvironment to improve cancer immunotherapy
Resistance to immunotherapy can also be in part explained by the regulation and nature of the complex immune system. Recently, we found that immunotherapies induce a reactive recruitment of immunosuppressive neutrophils into tumor tissue, thereby limiting therapeutic efficacy. This recruitment was dependent on the receptor tyrosine kinase c-MET and blocking of c-MET signaling enhanced the efficacy of immunotherapies in a variety of cancer models (Glodde et al., 2017).Neutrophils also appear to play a critical role for disease progression and T cell suppression in advanced ovarian cancer. However, the underlying mechanism how these or other myeloid immune cells contribute to immune regulation and disease progression in ovarian cancer is poorly understood.
By using orthotopic ovarian cancer models carrying human-relevant mutations, we aim to provide a deeper understanding of myeloid pathobiology in order to identify and develop novel targeted and personalized treatment strategies for microenvironmental reprogramming in combination with immunotherapies.
Developing novel immunotherapies for the treatment of ovarian cancer
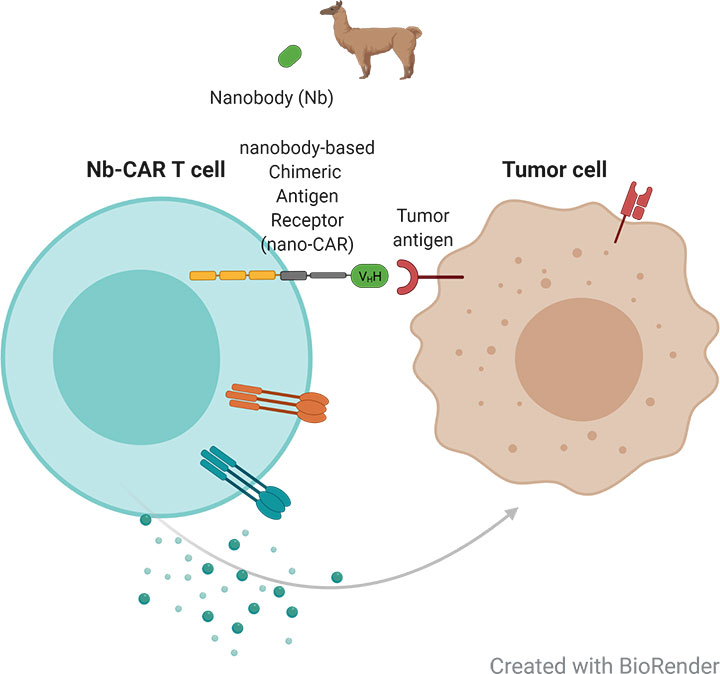
Chimeric antigen receptor (CAR) T cell therapy has emerged as an alternative to the blockade of immune checkpoint molecules and have shown promising results for the treatment of hematological malignancies, but are less effective for the treatment of solid tumors.CAR T cells based on nanobodies are smaller, have higher stability and serve as an ideal antigen recognition domain. In collaboration with the Nanobody Core Facility, we aim to establish a generic platform of nanobody-based CAR T cells. This will enable us to identify relevant target proteins, investigate the therapeutic efficacy in vitro and in ovarian cancer mouse models and thereby study resistance mechanisms, and complex tumor- and immune cell interactions.
Want to find new ways to improve immunotherapy?
Interested in more information about our projects?
Further Reading
Adoptive T Cell Therapy Targeting Different Gene Products Reveals Diverse and Context-Dependent Immune Evasion in Melanoma.
Effern M, Glodde N, Braun M, Liebing J, Boll HN, Yong M, Bawden E, Hinze D, van den Boorn-Konijnenberg D, Daoud M, Aymans P, Landsberg J, Smyth MJ, Flatz L, Tüting T, Bald T, Gebhardt T, Hölzel M
Immunity. 53, 564-580.e9 (2020).
Immunity. 53, 564-580.e9 (2020).
Experimental and stochastic models of melanoma T-cell therapy define impact of subclone fitness on selection of antigen loss variants” (preprint, Systems Biology, 2019)
Glodde N, Kraut A, van den Boorn-Konijnenberg D, Vadder S, Kreten F, Schmid-Burgk JL, Aymans P, Echelmeyer K, Rumpf M, Landsberg J, Bald T, Tüting T, Bovier A, Hölzel M
Nature comm, in rev. doi:10.1101/860023.
Nature comm, in rev. doi:10.1101/860023.
Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy.
Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, Hinze D, Rogava M, van der Sluis TC, Ruotsalainen JJ, Gaffal E, Landsberg J, Ludwig KU, Wilhelm C, Riek-Burchardt M, Müller AJ, Gebhardt C, Scolyer RA, Long GV, Janzen V, Teng MWL, Kastenmüller W, Mazzone M, Smyth MJ, Tüting T, Hölzel M
Immunity. 47, 789-802.e9 (2017).
Immunity. 47, 789-802.e9 (2017).